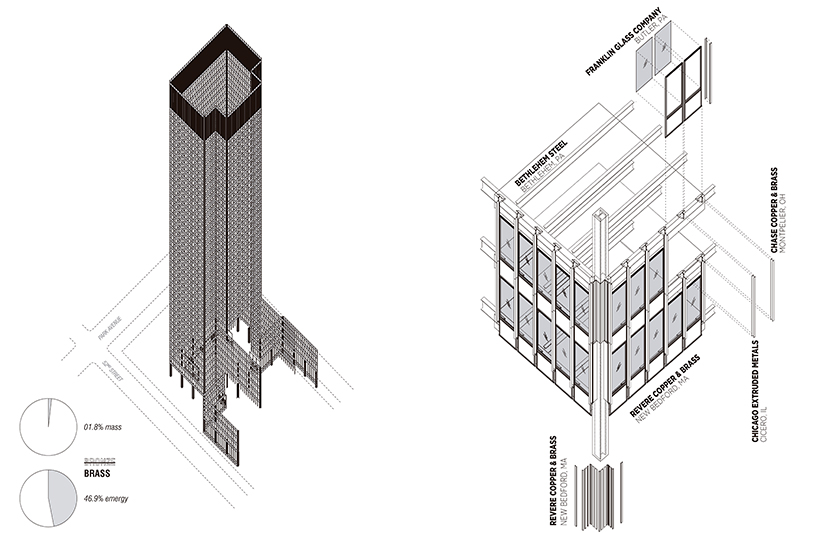
The first thing to know about the 3.2 million pounds of “bronze” technomass in the Seagram Building is that it is not bronze. It is brass. Brass is an alloy of copper and zinc. What the building industry refers to as bronze—C365 Architectural Bronze—is a specific brass alloy (56% copper, 41.5% zinc, 2.5% lead). When the alloy contains around 60% copper, it attains a reddish hue, a property that is lost at much higher or lower concentrations of copper-based alloys. In the case of the Seagram Building and its lower copper content, so as to obtain a deeper hue, the brass was “hand-rubbed”—and continues to be hand-rubbed annually—to attain and maintain the desire darkened appearance typically associated bronze.
In normal architectural descriptions and histories, the distinction between brass and bronze might seem to be a semantic or didactic distinction, a trivial detail perhaps. But in a terrestrial description of the Seagram Building, it is a difference that makes a difference. Actual bronze is also a copper alloy, though alloyed with a nominal amount of tin (12%) rather than the 42% of zinc in brass “architectural bronze.” While copper remains a constitutive component of both bronze and architectural bronze, if the Seagram Building were actually bronze, the emergy associated with the metallic enclosure would be about 231% higher than the brass/architectural bronze.
The high emergy-mass ratio observed in the chapter on Construction Ecology suggested that the architectural bronze reflected highly concentrated bio-geophysical resource accumulations, as well as significant transformations of those resources. As such, to track both the constitutive bio-geophysical inputs of the architectural bronze is to more fully gauge the telluric work attached to the Seagram Building’s enclosure, as well as the processing of those inputs through labor and machine. This begins to connect the work of the world and humans as part of a terrestrial description of the Seagram Building. But it will also reveal along the way the type of unequal economic and ecological exchanges that are inherent to these processes. In the course of this chapter, I will treat the architectural bronze that encloses the Seagram Building less as a “building material” and more as a terrestrial artifact, the technofossil evidence of ecological and social entanglements that we call building.
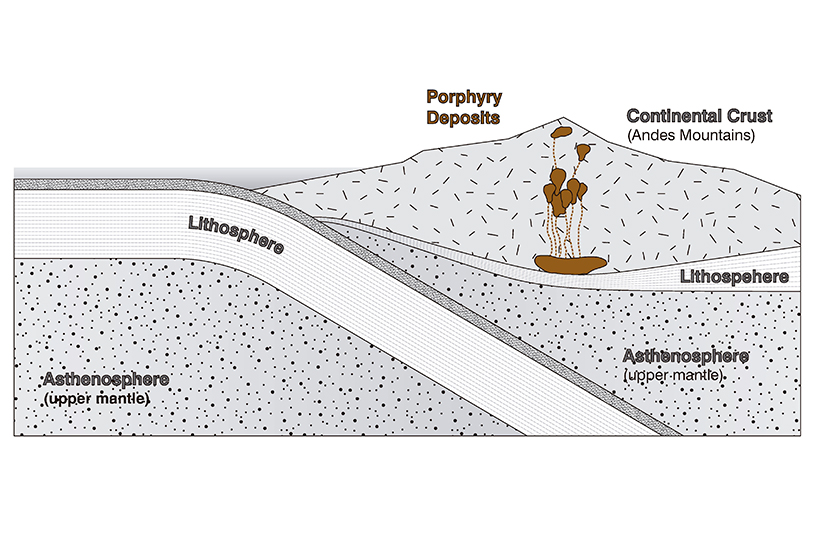
The basis of any terrestrial description of architecture is, again, that all architecture is geological. The system of engenderment for all architectural materials begins with the tectonics of the super-longue durée terrestre of geological history. The active and continuous convection of the solid material we consider to be fixed ground—a ceaseless planetary flow of plate materials sliding by each other, of heated material plumes rising up, of plates pushed, folded back down, and so on—yield the geographically-specific pockets of minerals that are the basis of all building materials. The statistically significant base chemical elements of architectural bronze are copper and zinc. The first step in describing this bronze as a terrestrial artifact is to grasp the emergence of these two chemical elements as geological phenomena. The copper and zinc deposits that eventually end up in buildings are found in porphyry deposits. Such deposits occur in zones of tectonic plate convergence, specifically where oceanic plates slide under continental plates; a process called subduction.
The friction of the subducting plate causes its surface to melt, resulting in an upward hydrothermic flow. The liquid magma thus rises with tremendous pressure up into the continental plate through fissures in its mantle. In the process of this convection, the magma cools but metallic portions of the flow often continue to rise to the surface, engendering the subsurface porphyry deposits and veins that humans mine. The geological result is an “epigenetic” deposit, meaning that the mineral deposits are much younger than the fissured strata of rock that the mineral deposit veins occupy. Indeed, the terrestrial base minerals of the Seagram Building envelope were formed hundreds of millions of years ago. What humans think of as metallic minerals are but a cooled flow, a hardened plenum, of tectonic activity; the entropic side-effect of tectonic activity. Eventually a similar mixture of heat, pressure, and flow would reshape the minerals into familiar Seagram Building formats in factories in Cicero, Illinois, Montpelier, Ohio, and New Bedford, Massachusetts. But first, a whole other set of terrestrial processes preceded the transformation of the copper and zinc that would become the much photographed and discussed “bronze” extrusions that enclose a most expensive skyscraper.
In the case of copper and zinc, both are typically extracted through open-pit bulk mining operations. This process involves the removal of tremendous amounts of material, so as to extract the veins and deposits of the desired mineral. Once bulk-mined, the extracted ore is processed, generally with abundant water and chemical processes, to separate the relatively small percentage of mineral (in the range of single-digit percentages) from the less desirable rock material stratum. The discarded rock material is referred to as tailings and constitutes the bulk of a mine’s material flow.
Prior to World War II, the United States was a net exporter of copper. The war armament effort, however, shifted the United States to a net importer of copper by 1940. Given the geopolitical disruptions in other hemispheres at this time, South America was the obvious source of imported copper. Chile, for example, supplied nearly half of the imported copper during this period (46%), followed by Canada
(14%), and Mexico (10%). By the mid-1950s, more than 50 percent of the refined metal [copper] continued to come from Chile. The award of a citation in 1956 recognizing the Seagram Building as “an outstanding and original contribution to world architecture embodying copper, one of Chile’s primary natural resources,” represents a significant endorsement and link to the Chilean sources of copper. The remaining copper for the building’s brass would have come from domestic sources. Of this domestic extraction, around 78 percent was open-pit mined and would have occurred in Montana, Michigan, Texas, Colorado, Utah, and Arizona.
The specific flow of copper that constitutes the Seagram Building is impossible to deduce, however. “Virgin” copper stock material may have been used, but according to Davis Anderson, who owned and operated the Cicero factory that produced brass components for the Seagram Building, most of the copper used in the production of the brass envelope would have been from recycled stock. Varied recycled copper stock would have been melted down, other materials and minerals removed, and billets of the C365 Architectural Bronze alloys produced for the project at each production facility.
According to metallurgical consultant, Lou Lozano, Anaconda American Brass Company supplied most of the copper for the Seagram Building project. It was part of a larger conglomerate of Anaconda companies involved in mining and metal production at the time. This corresponds with varied advertisements as well as an acknowledgement in a catalog for a Museum of Modern Art exhibition in 1957 called “Buildings for Business and Government,” which featured models and drawings of the Seagram Building.
To provide a sense of the scope of their operations, one report on the copper industry at the time described Anaconda as “engaged in mining, milling, and smelting nonferrous metal ores; refining and selling the metals obtained from these ores; fabricating semi-finished and finished copper and brass products; producing and fabricating aluminum; mining and processing uranium and manganese ores; and recovering, treating, and selling byproduct metals. The principal metals recovered from ores treated are copper, lead, and zinc; however, silver, gold, arsenic, cadmium, chromium, vanadium, selenium, and tellurium, also are recovered.
While the copper in the C365 Architectural Bronze billets would have likely come from a range of sources—mainly recycled scrap sources—as part of the world-systems analysis of copper technomass and technofossils, it is important to consider more generally and emblematically the types of sources for the copper in the Anaconda supply chain. Given the statistics on copper extraction cited above, it is most likely that the Seagram Building copper emerged from both domestic and international open-pit mines.
ANACONDA – BUTTE, MONTANA
One emblematic mine that was an active source of domestic copper extraction in the period leading to the Seagram Building production is the Anaconda Copper Mine in Butte, Montana. A civil war veteran established the mine in 1881. The original mine ceased operations in 1947. Anaconda operated other open-pit mines in the area and in 1955 it opened what is now known as the Berkeley Pit. A billion tons of material—300 million tons of ore and 700 million tons of tailings—were extracted from Berkeley. Its operations obscured the original Anaconda Copper Mine. The pit, now a mile-and-a-half by one mile in area, is nearly 1,800 feet deep. Half of this depth is now filled with water saturated with copper, iron, arsenic, cadmium, zinc, and sulfuric acid. Butte, Montana is now one the largest Superfund sites in the United States.
ANACONDA – CHUQUICAMATA, CHILE
By volume, the open-pit mine in Chuquicamata, Chile is the largest of its kind in the world. It is 2 miles long, 2.8 miles wide, and has a depth of 2,790 feet. Nearly 10,000 feet above sea level, Chuquicamata is located in one of the driest deserts on the planet, the Atacama. The dry, high-altitude climate makes for notorious working and living conditions. Its mining history dates back to Incan groups who surface mined materials for weapons. “Chuquicamata” means “tip of the spear” in their language. In the early nineteenth century, Spanish and English companies were engaged in surface mining at this site. It was not until the twentieth century, once a new method of process low-grade copper was developed, that mining operations at Chuquicamata escalated.
In 1911, Daniel Guggenheim established the Chile Copper Company, later the Chile Exploration Company, or Chilex, to develop the mine at Chuquicamata. The development of the mine required intense infrastructure including railroads, power plants, and a company town which consisted of “barracks, two public schools, a Court House, a telegraph and postal building, a theater, a hospital, a public quarantine, a Protestant church and a Catholic church, a music hall for workmen, and a public library.” Steam shovels from the Panama Canal were originally used as part of the mining operations. The first mining infrastructure, operational in 1915, would produce some 120,000,000 pounds of copper annually. Production volume would double by the end of that decade. Smelting was not initially used to refine the copper. Instead, oxidized copper sulfate ore was leached with dilute sulfuric acid and the copper was then collected on cathodes in large vats.
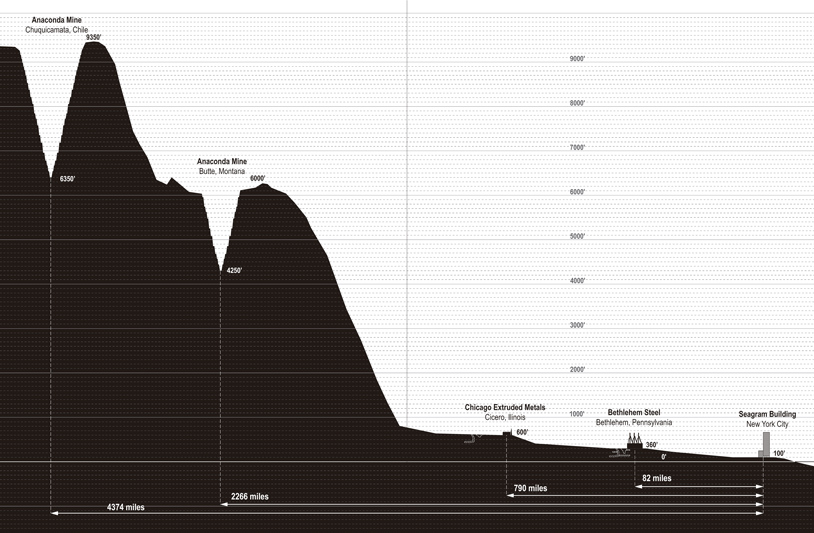
In 1923, the Anaconda Copper Mining Company purchased the mine from the Guggenheim consortium for $77 million and continued to expand the mine. The same year, Anaconda purchased the American Brass Company, the firm that would supply the Seagram Building with the copper for its brass alloy envelope. At that time, in 1955, the company name was changed to the Anaconda Company. In this decade, it would invest more than $100 million into the Chuquicamata mine. The 1957 discovery of the largest deposit of copper in the world, in what became the South Mine at Chuquicamata, was a further boon for mining operations. The company town adjacent to the mine was then home to 24,000 people.
In 1952, Ernesto “Che” Guevara visited the Chuquicamata mine early in his journey around South America. He was moved by his encounter with a married couple headed to labor in sulphur mines in the area “where the climate is so bad and the living conditions so hard that you don’t need a work permit and nobody asks you what your politics are.” By the time Che visited the Chuquicamata mine, it was producing 20 percent of the world’s copper and a strike was imminent. Tensions between the North American managers of the mine and the Chilean laborers was high. He notes that,
… an economic and political battle is being waged in Chile between a coalition of nationalist and leftwing groupings that advocate nationalizing the mines, and those who, in the cause of free enterprise, prefer a well-run mine (even in foreign hands) to possibly less efficient management by the state. Serious accusations have been made in congress against the companies currently exploiting the concessions, symptomatic of the climate of nationalist aspiration surrounding copper production.
In 1971, Chilean president Salvador Allende appropriated the Chuquicamata mine from Anaconda. The nationalization of this mine shifted operations to the National Corporation of Copper, known as Codelco. In terms of underdevelopment and unequal exchanges, this is a momentous shift in who benefits from extraction and how. When President Allende was ousted in 1973, Augusto Pinochet paid the Anaconda Company $250 million in compensation for Allende’ appropriation of the mine.
In 2004, the remaining 2,500 inhabitants were forced to move from the ninety-year-old company town adjacent to the mine.16 Four families a day were moving to the nearby town of Calama. Chuquicamata families had begun moving there as early as the 1970s, signaling the decline of Chuquicamata from its heyday in the mid-1950s. At that time when families were forced to move, the Chuquicamata mine was “the largest open-pit copper mine in the world, owned by Codelco and operating at a rate approaching one million tons of ore and waste every day.”17 A million pounds of waste tailings piles up quickly. As the open pit grew bigger and deeper, so did the spread of tailings around the mine. They began to encroach on the company town. In one of the most direct and visceral contradictions of capital, the waste stream of the mine literally began to crush the hospitals and houses of the workers in the mine. The urban atrophy of a mountain of tailings crushing its own mining company town is one of the most direct examples of underdevelopment one could imagine. It is also one of the most emblematic examples of technomass that one could imagine.
Part of the impetus for moving the town is that caustic arsenic and sulfur dioxide emissions from its smelting operations exceeded new environmental policies. While these policies undoubtedly placed new emphasis on the health of laborers, they also point to an admission of ninety years of polluted habitats for workers in the area.18 A celebration on September 1, 2007 marked the closure of Chuquicamata, a once-powerful company town-turned-dump, now buried under the mine’s tailings.